The following information on using lime on soil comes from Five Acres and Independence by M. G. Kains. Five Acres and Independence is also available to purchase in print.
Various materials are called “indirect” fertilizers or “amendments” because, though they may or may not contain plant food they are used chiefly to make changes in the soil and thus help plants to get food already present but in otherwise unavailable forms. The most important is lime.
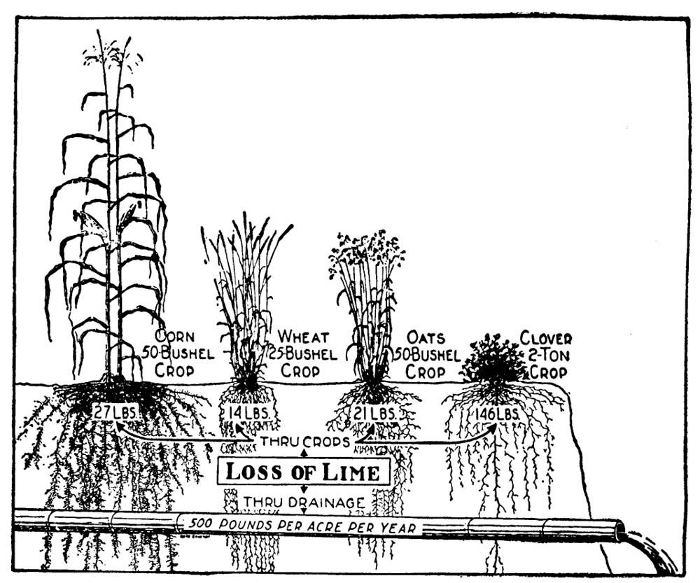
Lime is an alkali, a chemical which has the power to combine with an acid to form a “salt.” It is therefore used as a soil amendment chiefly for this purpose and thus to maintain the soil in “neutral” or slightly alkaline condition. If we were to use only natural manures our soils would continue favorable to plant growth much longer than when we use chemicals that contain acids (especially hydrochloric and sulfuric) which we apply whenever we fertilize with muriate or sulfate of potash or with sulfate of ammonia.
When we use such fertilizers we must sooner or later use lime to neutralize the acids they contain. The salts formed by these acids with the lime (chloride of lime and sulfate of lime—or gypsum), being highly soluble in water, are washed out of the soil in the drainage water, thus preventing damage these two powerful acids would do to soils and plants. Other acids are similarly neutralized by lime.
When land is cultivated and fertilized but yields only 75%, 50%, or 30% of a full crop it is like running an automobile with its wheels off the ground—it doesn’t get anywhere! Soil, fertilizer, and water are all present and labor is expended but acidity prevents fruition.
Although experimental work in many states indicates that most vegetable crops do best on neutral or only slightly acid soils, the New Jersey Station felt that further information would be valuable, so field plots where lime has been used in varying amounts at five-year intervals for 20 years were chosen for gathering data. Two kinds of limestone were used—calcium and magnesium—both finely ground and applied broadcast shortly before seeding. Without going into the details of Bulletin 498, all of which were identical except for the lime, let it here suffice that these experiments prove that:
In many cases labor and fertilizer are wasted because productivity capacity is distinctly lowered by the acid condition of the soil; that in every case the yields were much increased where lime was used; that beets and carrots were a failure on the unlimed plot, partly because acidity reduced germination; that poor germination of seeds due to soil acidity delays marketing or even results in loss of crop; that, because of its value in growing soil-improving crops, lime may have an important indirect effect, because most of these crops do best on soils only slightly acid; that certain soil organisms which play important parts in soil fertility are greatly favored by a soil well supplied with lime; that strongly acid soils may contain soluble aluminum compounds which are toxic to certain crop plants, whereas lime puts these compounds out of action; that heavy applications of superphosphate will do the same, but lime is more economical; and that when used in moderate amounts, there is little choice between the magnesian and the nonmagnesian limestone.
Gypsum or land plaster cannot be used to neutralize acids because it is already a salt (sulfate of calcium). It may be used, however, for the other purposes for which lime is employed so it is safer to apply when a soil is already neutral or slightly alkaline and therefore not in need of the caustic form of lime.
Several fairly accurate apparatuses to determine whether a soil is acid, neutral or alkaline are for sale at garden supply stores. For practical purposes, however, the following ways will answer:
1. Shake a sample of soil with some rain or distilled water in a bottle, allow the sediment to settle, dip strips of druggists’ red and blue litmus paper in the water and note any change of color. If the red paper turns blue the soil is alkaline; if the blue paper turns red the soil is acid; if neither paper changes much it is neutral.
2. Instead of the litmus test add a teaspoonful of weak ammonia (not the “cloudy” household stuff but pure ammonia in water) to the muddy water. After standing over night if the liquid has turned dark brown or black the soil is acid; if not it is neutral or alkaline.
Govern your applications of lime or gypsum by what several such samples of soil taken from various parts of the field indicate.
Because of its caustic nature lime must never be brought in direct contact with manure. Its chemical action drives the ammonia of the manure into the air. We need every bit of this ammonia for our plants so should try to prevent its loss by dusting the manure with some absorbent or by burying it in the soil as soon as possible. Among the best absorbents are superphosphate, gypsum, dry muck and shredded peat moss. These may be scattered freely over the manure as made.
Wood ashes should be handled in the same way as lime because, though they contain all the mineral elements of plant food, they are noted for their potash and lime content and the effects of the latter upon soils. Never should they be mixed with manures.
As lime and gypsum always work downward in the soil from the level of their application it is important to apply them after the ground has been dug or plowed and to rake or harrow them in the surface. If they are applied before plowing they work downward from the level of the inverted soil and are therefore of no use in the surface layer. When these materials are to be used at the same time as manure the manure should be applied before plowing or digging and the lime or gypsum afterwards.
The amount of lime to apply will vary with the character of the soil and the degree of acidity. For light sandy loams an application of 1,000 to 2,000 pounds of pulverized limestone or 700 to 1,500 pounds of slaked lime (hydrate) to the acre should be ample as a dressing unless the soil is decidedly acid; then double the quantity may be applied. For a heavy clay, 1,500 to 5,000 or 6,000 pounds of pulverized limestone or 1,300 to 5,000 pounds of slaked lime to the acre are liberal applications. As a rule it will not be necessary to apply lime oftener than once in 3 to 6 years.
Of the several forms in which lime may be bought the kind generally known as hydrated or agricultural is the most convenient. It is sold in paper bags, is finely pulverized and passes through one’s hand like melted butter. However, it may be more evenly applied by a fertilizer distributor.
