The following information commercial fertilizers and potash comes from Five Acres and Independence by M. G. Kains. Five Acres and Independence is also available to purchase in print.
In soils that have long been cultivated, especially in those which have been mismanaged, one or more of three or four essential elements of plant nutrition may be deficient. Hence we must resort to commercial fertilizers to supply the needs of our crops.
Commercial fertilizers are of two classes; organic (of vegetable and animal origin) and inorganic (of mineral origin). The former, which include manures, play their chief role in improving the physical condition of the soil, though they also supply important amounts of plant food; the latter play less important or (some of them even negative) physical roles, but are useful for their plant foods. The former have the advantage of ready conversion into available plant foods and of harmlessness to foliage upon which they fall. Some of their characteristics are as follows:
Dried blood, dried fish and cottonseed meal are all rich in nitrogen but also contain more or less potash and phosphoric acid; ground bone, chiefly noted for its phosphorus, also contains more or less nitrogen and potash; tankage, also noted for its phosphorus, but varying considerably in the amounts because of different materials from which made—fish, garbage, sewage, etc.—also contains potash and nitrogen.
The plant food most likely to be lacking in the soil, nitrogen, is the growth maker. Though it is only one of the essential elements of plant growth it is of special importance because its compounds are deficient in most soils, costly to buy, easily lost by drainage, and yet easy to replace by inexpensive methods. It constitutes about 80% of the air, but not until it becomes combined with other elements in such forms as ammonia and nitrates can most cultivated plants use it. Most of these combined forms, together with varying quantities of other combinations in organic compounds, are formed in the upper layers of the soil where plant and animal wastes accumulate. Such supplies are reduced more or less rapidly by cultivation, mismanagement, seepage to lower levels, and by escape into the air in gaseous forms.
In the presence of sunlight and when supplied with water, plants are able to get all their necessary plant food except nitrogen from the air and the soil. But without nitrogen they can neither grow nor even live. Growth, repair and reproduction are all directly or indirectly dependent upon nitrogen. One of the best indexes of its quantity in the soil is the character of the foliage. When this is lush and dark green the supply is ample or even excessive; when puny and yellowish, it is deficient. Rank stems and leaves are more susceptible to disease, slower to reach maturity and less hardy than those of only moderate growth.
The reason why nitrogen is often lacking in soils is that, being highly soluble, it is readily washed out of the soil and lost in the drainage water. Hence it is best applied in frequent but small doses during the first half of the growing period of the crop to be fertilized by it, never late in the growing season of shrubs and trees because this would probably result in sappy growth that might not ripen before winter and would probably be killed by freezing weather.
In addition to the supplies from manure and other organic materials, nitrogen is obtained in several chemical forms of which nitrate of soda and sulfate of ammonia are best known. The former, which contains about 15% nitrogen is quickly available to plants because of its ready solubility; hence it should be applied only in small doses at a time but repeated at intervals of two to four weeks; the latter, which contains about 20% nitrogen, is less quickly dissolved and is “fixed” or “held” much better in the soil so is less likely to be lost by leaching. It is therefore of special value in sandy and other light soils and in wet season under which conditions nitrate of soda would be quickly lost.
Nitrate of soda tends to make and maintain soils in neutral or alkaline condition—favorable to vegetables and most ornamental plants; sulfate of ammonia tends to make soil acid and therefore unfavorable to these plants but favorable to blueberries, rhododendrons and various other acid-tolerant plants. The acid reaction of this fertilizer may be neutralized by applications of wood ashes or lime. (Chapter 27.)
Potash, the fiber maker, is often lacking in sandy soils and in soils which have grown root crops (turnips, carrots, beets, parsnips, etc.) for several to many years without its adequate replacement in fertilizers or manures. When deficient the stems and branches of plants are weak and spindling and easily broken by wind.
Potash is obtainable from four main sources—wood ashes (about 4%), muriate of potash (50%), sulfate of potash (48%) and kainit (12% to 16%). Ashes contain all the mineral elements of the plants burned to make them. In order to be most useful they must be stored and applied dry. It will usually not pay to buy them but it will pay to use what supplies are made on one’s own place, dusting the material on the ground anywhere plants are to grow.
Reliance for a potash supply should be placed on the muriate or the sulfate (the former preferred) because these are high grade so need be bought and applied in only small amounts. Potash is not washed out of the soil in the drainage water but fixed or held by various materials in the ground. It may therefore be applied at any time of year when the ground is not frozen.
Phosphorus, the ripener, causes fruit and seed to ripen well. When lacking in the soil, crops may be slow to mature or may fail altogether. It is usually applied as superphosphate, basic (or Thomas) slag or pulverized phosphate rock (“floats”).
Though superphosphate (formerly called acid phosphate) has long been the leading phosphate fertilizer of America it is open to the objection that its analysis (17% to 20% of available phosphoric acid) is too low to meet the increasing demand for higher grade fertilizer mixtures; moreover, in humid weather it tends to cake because of its absorption of moisture from the air.
To offset these objections, manufacturers of mixed fertilizers add one or another of several concentrated materials to their goods. Doubtless the most popular of these is double superphosphate which is often marketed as “triple superphosphate” and “treble superphosphate.”
In physical properties and effects double superphosphate is on a par with the best of other concentrated inorganic fertilizers such as nitrate of potash, phosphates of ammonium and potassium. When properly prepared it neither absorbs moisture, cakes in storage nor becomes sticky even in humid air. Hence it enhances the drilling properties of fertilizer mixtures to which it is added and therefore makes them flow more freely through fertilizer drills.
Its solubility in water being only a tenth as great as other concentrated phosphate fertilizers is of great advantage because it is far less likely to burn the crops to which it is applied. Burning is due to highly concentrated solutions of soluble salts either upon the foliage or in the soil. When it replaces superphosphate in mixtures no undesirable mechanical or physical changes occur. However, one caution is necessary: it should not be mixed with excessive amounts of lime, ground limestone, cyanamid, or other materials rich in lime. When mixed with potash or ammonia or other soluble sulfates a chemical reaction occurs and results in more or less caking, but when this has completed itself the mixture is readily pulverized without recurrence of caking.
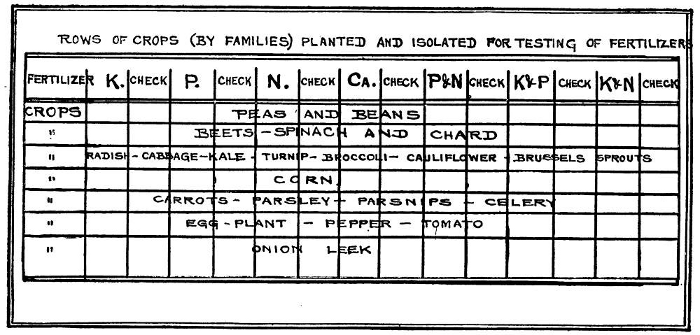
Before applying any of these fertilizers it is advisable to know which one is needed and which not; for thus we may avoid wasting materials and money. The simplest way to do this is to divide the garden into strips of at least 10 feet width at right angles to its length and sow only one unmixed fertilizer on each alternate strip, leaving the others unfertilized. Then sow crops lengthwise of the garden so as to cross the strips. (Fig 31.) When the crops grow the differences of development will suggest what plant foods are lacking in the soil and therefore what ones to supply. As already noted yellowish foliage will indicate shortage of nitrogen; weak stems, lack of potash; and poor ripening of fruit and seed, lack of phosphorus.
There should be at least five strips, one to test the need for nitrogen, another for potash and a third for phosphorus with blank, unfertilized strips between. Perhaps the best fertilizers to apply in the experiment are nitrate of soda for nitrogen; superphosphate for phosphorus and muriate of potash for potash. When enough space is available other strips may be added as follows: Sulfate of ammonia and sulfate of potash with unfertilized strips between. If desired, combinations of two or even three of these five fertilizers may be made thus: Nitrate of soda and superphosphate; nitrate of soda and muriate of potash; superphosphate and muriate of potash, etc., but not two of the same kind such as nitrate of soda and sulfate of ammonia or muriate of potash and sulfate of potash.
It is not considered advisable to divide complete* fertilizers into successful applications because abundant experiments have proved that the best time to apply potash and phosphoric acid is shortly before seed is sown or plants transplanted. Spinach, lettuce, celery, cabbage, cauliflower and other leafy vegetables are generally stimulated by top dressings of quickly available nitrogenous fertilizers, especially during chilly, wet spells when the rate of growth is slow. [*”Complete” fertilizers are “mixed goods” in which all three elements, nitrogen, potash and phosphorus, are included.]
Vegetables whose fruits are the parts wanted—melons, cucumbers, eggplants and tomatoes—are often helped by an application of nitrate of soda just as the first flower buds develop. Root crops do not respond so strikingly to surface applications, doubtless because they forage more deeply than do the other crops mentioned. Top dressings usually range from 150 to 300 pounds to the acre, the lower amount being applied, generally, all at one time, the larger in two dressings. In cases of larger total applications it is advisable to make fractional ones at 2-week intervals.
A good general formula for an ordinary fertile soil well managed as already explained is made of high grade materials, in percents, as follows: Nitrate of soda, 5%; sulfate of ammonia, 10%; dried blood, 15%; muriate of potash (or sulfate), 15%; superphosphate or ground bone, 55%, (the former preferred where quick availability is desired, the latter where a long effect is wanted). Its application should be followed in the vegetable garden by one to three surface dressings of nitrate of soda at intervals of three or four weeks.
Fertilizer efficiency may be increased by assuring uniformity of distribution, placing it where it will be of greatest service to the seeds or plants without risk of burning to roots, and by using the best available fertilizers.
Though fertilizer distributing machines are time and labor savers they often fail to apply at least some kinds of fertilizer evenly—too little resulting in insufficiently fed plants; too much in burning and killing. Such irregularity may be due to inefficiency of the machine, either because of poor design or working parts, to improper mixing or segregation of the ingredients, to stickiness, caking, moisture absorption or other cause that interferes with drillability.
Separation or segregation in mixtures may be due to size or to the specific gravity of the individual grains, the rate and amount varying with the materials. Granular and dry materials drill much better than damp and finely pulverized ones. Deliquescent materials—those that, like nitrate of soda, absorb moisture from the air—become caky or sticky so that their drillability may vary from day to day and thus necessitate frequent adjustment of the drill.
Numerous experiments have proved that fertilizer gives best results when placed more or less locally to the seeds or plants rather than scattered promiscuously over the surface, but that this distance should be relatively distant, not in contact with the seed or plants.
Other experiments have proved that granulating both soluble and insoluble fertilizers not only improves drillability by reducing the tendency to cake and become sticky but prevents segregation of the ingredients.
The only way to judge the value of a commercial brand of fertilizer is by its content of actual plant food. The laws of most states require that guaranteed analyses appear on the packages. When these are taken as the basis of calculation the values of various fertilizers can be determined and comparisons made to see which are the best ones to buy. Such calculations will usually show that pound for pound the concentrated or so-called high grade fertilizers, though naturally higher priced, are the more economical to buy, first because they contain larger percentages of the various plant foods and second because freight, cartage and application cost less for the smaller weights of material that need be handled to obtain given quantities of plant food.
Concentrated fertilizers and proprietary brands, especially the new synthetic nitrogen products, must be applied to soils with more caution than the low grade goods. In no case should the manufacturer’s recommendations be exceeded because these may be taken as maximum safe quantities. In order to avoid injury it will be advisable to apply less than the makers advise so as to be on the safe side.
The whole problem of how much fertilizer to use for a crop, and what formula of nitrogen, phosphorus, and potash, is tremendously complicated. We do not understand as yet the part played by the minor trace elements: zink, boron, magnesium, copper, etc. If the farmer is raising several main cash crops, the logical procedure is to run a number of fertilizer experiments each year on his chief income producing lines. A small plot, alternate rows, or blocks can be used for experimentation. Make a record of the amounts and types of fertilizer used. Do not try to depend upon memory when checking the results at harvest time. Over a period of a few years, one can learn much about the food requirements of different crops. Soils vary greatly; fields on the same farm vary in composition, humus, and availability of chemicals. A farmer who will experiment with fertilizers on his own soils will discover facts that eventually will help raise his income level.
